37 pv diagram problems and solutions
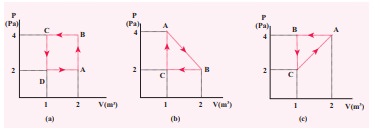
Isothermal thermodynamic processes – problems and solutions. 1. PV diagram below shows an ideal gas undergoes an isothermal process. Calculate the work is done by the gas in the process AB. Solution. Work done by a gas is equal to the area under the PV curve. AB = triangle area + rectangle area. W = [½ (8 x 10 5 –4 x 10 5)(3-1)] + [4 x 10 ... 10 Sep 2018 — Cyclic processes · The PV diagrams for a thermodynamical system is given in the figure below. · In the case (a) the closed curve is anticlockwise.
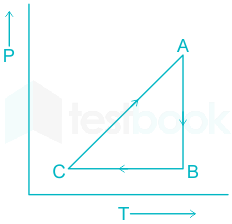
A cyclic Process ABCA as shown in below V-T diagram is performed with a constant mass of ideal gas.Show the process in the P-V digram. Solution. A straight line between A to B on V-T diagram indicates V αT V α T .So Pressure is constant. Volume is constant from B-C .Now since temperature is decreasing, Pressure must decrease.

Pv diagram problems and solutions
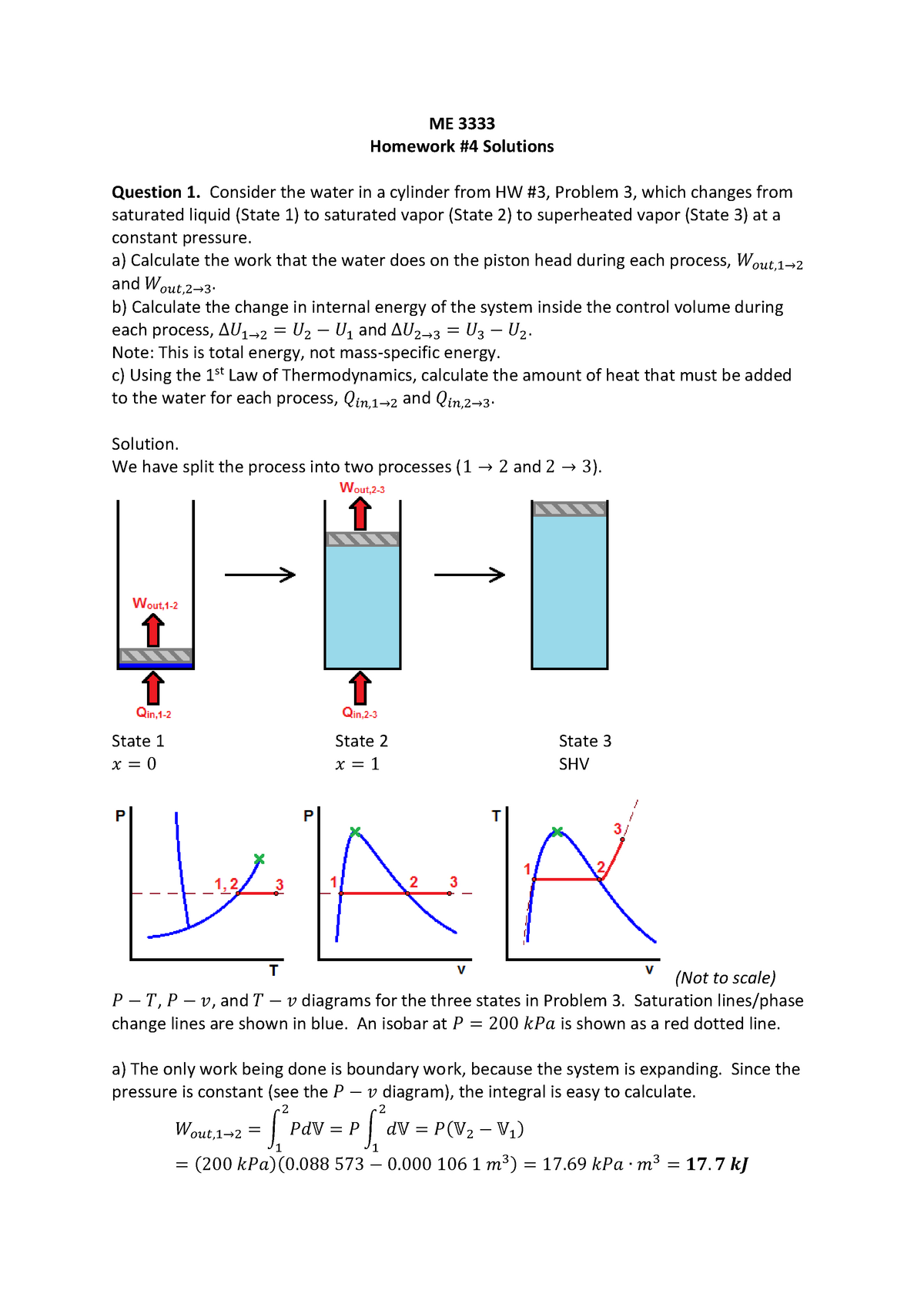
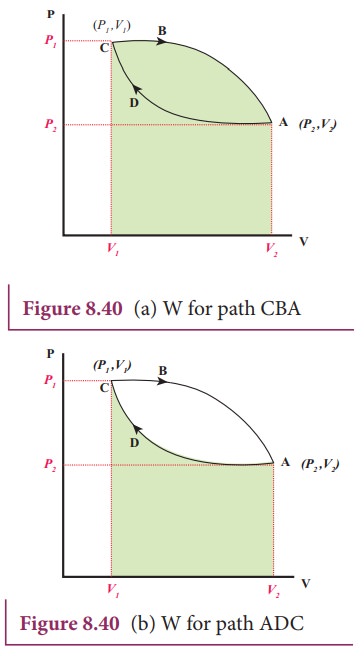
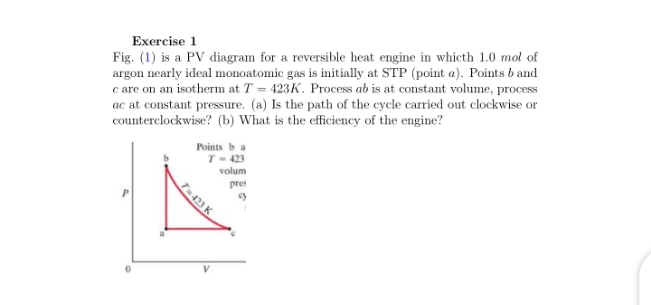
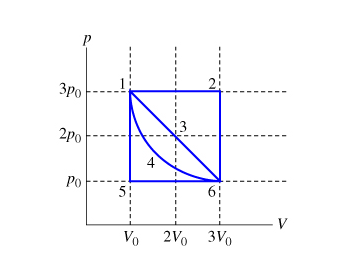
Problems practice. One mole of an ideal, monatomic gas runs through a four step cycle. All processes are either isobaric or isochoric. The pressure and volume of the gas at the extreme points in the cycle are given in the table below. Sketch the PV graph of this cycle. Determine the temperature at state A, B, C, and D. ACT 1: Solution Consider the two systems shown to the right. In Case I, the gas is heated at constant volume ; in Case II, the gas is heated at constant pressure . Compare Q I, the amount of heat needed to raise the temperature 1ºC in system I to Q II, the amount of heat needed to raise the temperature 1ºC in system II. A) Q I< Q II B) Q I= Q ... EXAMPLE 8.15 (PV diagram) A gas expands from volume 1m3 to 2m3 at constant atmospheric pressure. (a) Calculate the work done by the gas. (b) Represent the work done in PV diagram. Solution (a) The pressure P = 1 atm = 101 kPa, V f =2 m 3 and V 1 = 1m 3. From equation (8.17) W = Since P is constant. It is taken out of the integral.
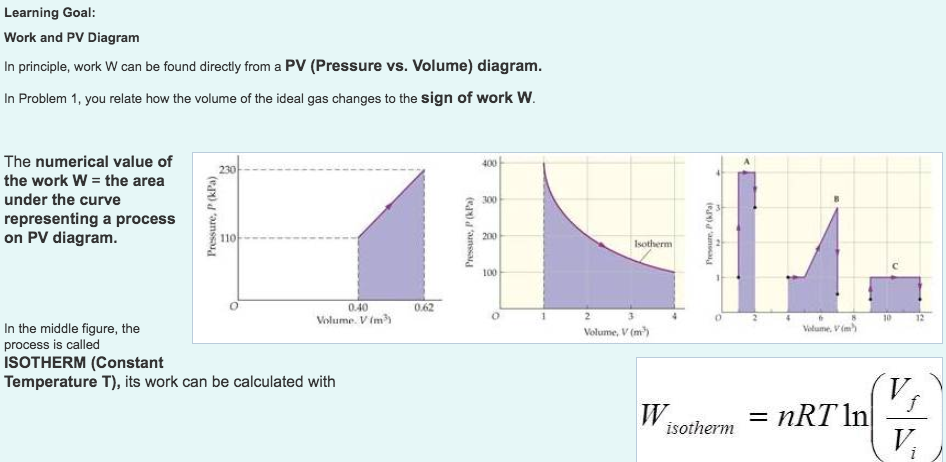
Pv diagram problems and solutions. Problem #6: A 12.0 g sample of gas occupies 19.2 L at STP. What is the molecular weight of this gas? Solution: This problem, as well as the two just above can be solved with PV = nRT. You would solve for n, the number of moles. Then you would divide the grams given by the mole calculated. 1) Use PV = nRT: (1.00 atm) (19.2 L) = (n) (0.08206) (273 K) EXAMPLE PROBLEMS AND SOLUTIONS A-3-1. Simplify the block diagram shown in Figure 3-42. Solution. First, move the branch point of the path involving HI outside the loop involving H,, as shown in Figure 3-43(a).Then eliminating two loops results in Figure 3-43(b).Combining two look at PV diagrams. A PV diagram is a graph of Pressure as a function of Volume. There are four different situations that you can expect to see shown in PV diagrams: 1. Isobaric: the gas is held at a constant pressure 2. Isochoric: the gas is held at a constant volume 3. Isothermal: the gas is held at a constant temperature 4. Sketch the cycle path on a PV Diagram b.) Calculate the net work done in kJ Data: T 1 = 145 o C, T 2 = 440 o C, P 1 = 150 kPa : Read : Work your way around the cycle, step by step. The work for the cycle is the sum of the work for each step. Assume the CO 2 behaves as an ideal gas throughout all three process steps.
A convenient way to visualize these changes in the pressure and volume is by using a Pressure Volume diagram or PV diagram for short. Each point on a PV diagram corresponds to a different state of the gas. The pressure is given on the vertical axis and the volume is given on the horizontal axis, as seen below. EXAMPLE 8.15 (PV diagram) A gas expands from volume 1m3 to 2m3 at constant atmospheric pressure. (a) Calculate the work done by the gas. (b) Represent the work done in PV diagram. Solution (a) The pressure P = 1 atm = 101 kPa, V f =2 m 3 and V 1 = 1m 3. From equation (8.17) W = Since P is constant. It is taken out of the integral. ACT 1: Solution Consider the two systems shown to the right. In Case I, the gas is heated at constant volume ; in Case II, the gas is heated at constant pressure . Compare Q I, the amount of heat needed to raise the temperature 1ºC in system I to Q II, the amount of heat needed to raise the temperature 1ºC in system II. A) Q I< Q II B) Q I= Q ... Problems practice. One mole of an ideal, monatomic gas runs through a four step cycle. All processes are either isobaric or isochoric. The pressure and volume of the gas at the extreme points in the cycle are given in the table below. Sketch the PV graph of this cycle. Determine the temperature at state A, B, C, and D.






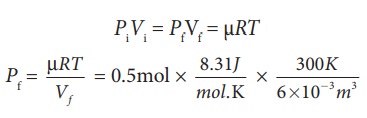










0 Response to "37 pv diagram problems and solutions"
Post a Comment